Lead-Acid vs. Lithium-Ion Batteries: The 3 Critical Differences You Must Know
Lead-Acid vs. Lithium-Ion Batteries: A Comprehensive Comparison
In the world of energy storage, two battery technologies have dominated the landscape for decades: the traditional lead-acid and the modern lithium-ion. Each possesses unique characteristics that make them suitable for different applications.
The first lead-acid battery was invented in 1859 by French physicist Gaston Planté, making it the oldest rechargeable battery technology still in use today. Despite its age, this technology has evolved and remains relevant in many applications.
Lithium-ion batteries, on the other hand, are relatively newcomers, commercialized in the early 1990s. They represent a more modern approach to energy storage and have gained tremendous popularity in recent decades.
How They Work: Fundamental Differences
Lead-acid batteries generate electricity through a chemical reaction between lead dioxide (PbO₂), sponge lead (Pb), and sulfuric acid (H₂SO₄) electrolyte. When discharging, both electrodes turn into lead sulfate, and the sulfuric acid becomes primarily water. The reaction reverses during charging.
Lithium-ion batteries operate on the movement of lithium ions between anode and cathode through an electrolyte. The anode is typically made of graphite, while the cathode consists of various lithium metal oxides. During discharge, lithium ions move from anode to cathode, and this process reverses during charging.
Comparative Analysis: Advantages and Disadvantages

To better understand how these battery technologies compare, let’s examine their key characteristics side by side:
| Characteristic | Lead-Acid Batteries | Lithium-Ion Batteries |
|---|---|---|
| Energy Density | Low (∼40 Wh/kg) | High (∼150 Wh/kg) |
| Cycle Life | 200-300 cycles | 500-1000+ cycles |
| Cost | Lower initial cost | Higher initial cost (∼3× more) |
| Weight | Heavy | Light (1/3 of lead-acid) |
| Efficiency | 80% discharge efficiency | >90% discharge efficiency |
| Charging Time | 6-8 hours | 2-4 hours |
| Temperature Performance | Good (-40°C to 60°C) | Reduced in cold conditions |
| Safety | Stable, non-flammable electrolyte | Thermal runaway risk |
| Environmental Impact | Recyclable but contains toxic lead | Fewer toxins but recycling challenges |
Advantages of Lead-Acid Batteries
- Proven Technology and Reliability: With over 150 years of development, lead-acid batteries represent a mature technology known for its stability and reliability. They perform well across a wide temperature range from -40°C to 60°C.
- Cost Effectiveness: Lead-acid batteries are the most affordable secondary battery technology available1. Their initial cost is approximately one-third that of lithium-ion batteries.
- High Recyclability: Lead-acid batteries boast an impressive recycling rate—over 98.5% in the United States and above 90% in China. The recycling process is well-established and economically viable.
- Safety Advantages: With non-flammable electrolyte and robust construction, lead-acid batteries pose minimal fire risk compared to lithium-ion alternatives1. They’re suitable for high-vibration applications like vehicle starting systems.
- High Power Delivery: These batteries excel in providing high surge currents, making them ideal for applications like engine starting where brief, high-power bursts are required.
Disadvantages of Lead-Acid Batteries
- Low Energy Density: The bulky and heavy nature of lead-acid batteries makes them unsuitable for portable applications where weight and space are constraints13. Their energy density is only about one-third that of lithium-ion batteries.
- Limited Cycle Life: With typically 200-300 deep cycles before significant capacity degradation, lead-acid batteries require more frequent replacement than lithium-ion alternatives in cyclic applications.
- Maintenance Requirements: Flooded lead-acid batteries need regular maintenance including water topping-up and terminal cleaning to ensure longevity and performance.
- Slow Charging: Lead-acid batteries require 6-8 hours for a full charge, significantly longer than the 2-4 hours needed for lithium-ion batteries.
- Environmental Concerns: The use of lead, a toxic heavy metal, raises environmental concerns throughout the battery lifecycle from production to disposal.
Advantages of Lithium-Ion Batteries
- High Energy Density: Lithium-ion batteries provide approximately three times the energy density of lead-acid batteries by both weight and volume. This makes them ideal for portable applications.
- Long Cycle Life: Capable of 500-1000+ charge cycles before significant degradation, lithium-ion batteries typically last 2-3 times longer than lead-acid equivalents in deep-cycle applications.
- Excellent Efficiency: With discharge efficiency exceeding 90%, lithium-ion batteries lose less energy as heat during charge and discharge cycles compared to lead-acid batteries’ 80% efficiency.
- Low Self-Discharge: Lithium-ion batteries maintain their charge for much longer periods when not in use, with self-discharge rates of just 2-5% per month.
- Minimal Maintenance: These batteries are essentially maintenance-free, requiring no periodic watering, equalization charges, or terminal cleaning.

Disadvantages of Lithium-Ion Batteries
- Higher Initial Cost: The substantial upfront investment required for lithium-ion technology remains a significant barrier for many applications.
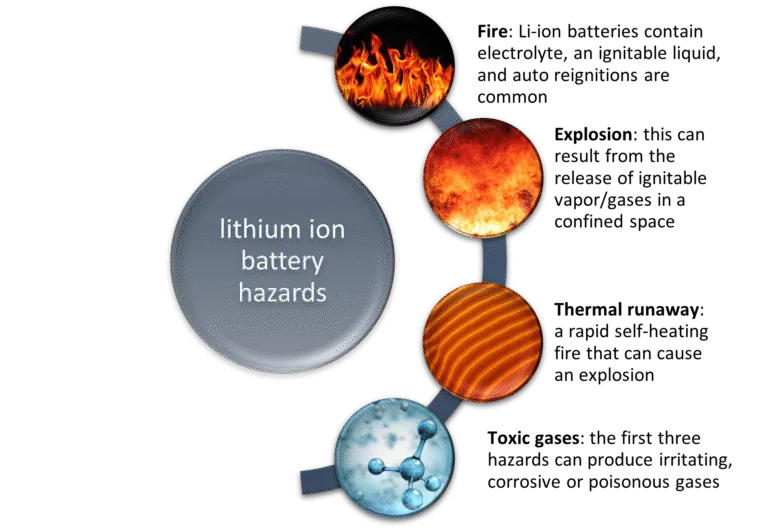
- Safety Concerns: Lithium-ion batteries carry risk of thermal runaway which can lead to fires or explosions in cases of overcharging, short-circuiting, or physical damage.
- Complex Battery Management: They require sophisticated management systems to monitor voltage, temperature, and current to ensure safe operation and prevent damage.
- Recycling Challenges: Despite containing fewer toxic materials, lithium-ion batteries have a recycling rate below 5% globally due to technical and economic constraints.
- Performance in Extreme Cold: Lithium-ion batteries experience significant capacity reduction in low-temperature environments, sometimes as much as 30-50% at -20°C.
Environmental Impact Comparison
The environmental implications of battery technologies extend across their entire lifecycle from material extraction to manufacturing, use, and ultimately disposal or recycling.
Lead-acid batteries contain toxic materials but enjoy a well-established recycling infrastructure that recovers about 90% of the lead for reuse110. However, lead pollution remains a concern throughout the产业链 (including primary lead smelting, battery manufacturing, battery recycling, and secondary lead smelting), posing potential environmental and health hazards if not properly managed.
Lithium-ion batteries contain fewer immediately toxic materials but present different environmental challenges. The extraction of lithium, cobalt, and other rare metals often involves water-intensive processes and can contaminate local ecosystems5. Currently, less than 5% of lithium-ion batteries are recycled globally due to technical complexities and economic barriers.
Application Considerations
When to Choose Lead-Acid Batteries:
- Stationary applications where weight is not a concern (UPS systems, backup power)
- Budget-conscious projects with limited initial capital
- High-vibration environments like automotive starting applications
- Situations where recycling infrastructure is well-established
- Applications requiring high surge currents for short durations
When to Choose Lithium-Ion Batteries:
- Portable applications where weight and size matter (electronics, EVs)
- Deep-cycle applications requiring frequent discharging
- Situations where minimal maintenance is essential
- Applications where long-term cost outweighs initial investment
- Off-grid systems where efficiency translates to smaller solar arrays

Future Developments
Both technologies continue to evolve. Advanced lead-acid batteries like lead-carbon and bipolar designs are addressing some traditional limitations regarding energy density and cycle life1. Similarly, lithium-ion technology is advancing with solid-state electrolytes promising enhanced safety and energy density.
The emerging battery recycling technologies aim to improve the sustainability of both battery types, with particular attention to developing more efficient lithium-ion recycling processes.
Conclusion
The choice between lead-acid and lithium-ion batteries involves balancing multiple factors including cost, weight, lifespan, application requirements, and environmental considerations.
For those prioritizing initial affordability, proven reliability, and established recycling, lead-acid batteries remain a compelling choice, especially for stationary applications where weight is not a constraint.
When energy density, longevity, efficiency, and lightweight properties outweigh initial cost concerns, lithium-ion technology typically delivers superior performance despite its higher upfront investment.
As both technologies continue to evolve, we can expect further improvements in performance, safety, and sustainability across the energy storage landscape, ultimately benefiting consumers and the environment alike.







2 Comments